UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): |
(Exact name of Registrant as Specified in Its Charter)
(State or Other Jurisdiction |
(Commission File Number) |
(IRS Employer |
||
|
|
|
|
|
|
||||
|
||||
(Address of Principal Executive Offices) |
|
(Zip Code) |
||
Registrant’s Telephone Number, Including Area Code: |
|
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
Securities registered pursuant to Section 12(b) of the Act:
|
|
Trading |
|
|
|
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item 2.02 Results of Operations and Financial Condition.
As discussed in Item 8.01 of this Current Report on Form 8-K, IGM Biosciences, Inc. (the “Company”) updated its corporate presentation and disclosed that the Company had an estimated cash and investments balance of $427.2 million as of December 31, 2022 (unaudited).
Item 8.01 Other Events.
The Company will participate in the 41st Annual J.P. Morgan Healthcare Conference in San Francisco, California from January 9-12, 2023.
A copy of the Company’s updated corporate presentation is attached hereto as Exhibit 99.1 and is incorporated by reference herein.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
Exhibit |
|
Description |
99.1 |
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
Date: |
January 6, 2023 |
By: |
/s/ Misbah Tahir |
|
|
|
Misbah Tahir |

Corporate Overview January 6, 2023 Global Leaders in IgM Antibodies Exhibit 99.1 Igm biosciences® REIMAGINING antibody medicines

Forward-looking statements . This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect the current views of the management of IGM Biosciences, Inc. (the “Company,” “we” or “our”) based on information available to us as of the date hereof. All statements other than statements of historical fact could be deemed forward-looking, including but not limited to statements regarding our unaudited cash and investments balance as of December 31, 2022; our future financial performance; plans, timelines, and expectations related to our preclinical studies, clinical trials, discovery programs and collaboration activities; business plans, strategies, strategic priorities, catalysts and objectives; our ability to obtain regulatory approval; the potential therapeutic benefits and economic value of our product candidates; potential growth opportunities; and our competitive position, industry environment and potential market opportunities. In some cases, you can identify forward-looking statements by terms such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potentially” “predict,” “should,” “target,” “will” or the negative of these terms or other similar expressions. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including , among other things: plans, timelines, and expectations related to our preclinical studies, clinical trials and our discovery programs including regarding the availability of data, planned regulatory filings, the initiation and progress of current and future clinical trials; potential delays and disruption resulting from the COVID-19 pandemic, including related supply chain disruptions and constraints; the risk of the occurrence of any event, change or other circumstance that could give rise to the termination of collaborations with third parties, including the agreement with Sanofi; our early stages of clinical drug development; our ability to achieve clinical goals; risks related to the use of engineered IgM antibodies; our ability to utilize our lgM antibody platform to generate and advance additional product candidates; our ability to advance product candidates into, and successfully complete, clinical trials; our ability to adequately demonstrate sufficient safety and efficacy and reduced toxicity, of our product candidates, either alone or in combination with other compounds; the potential for the results of clinical trials to differ from preclinical, preliminary, initial or expected results; the risk of significant adverse events, toxicities or other undesirable side effects; the timing or likelihood of regulatory filings and approvals; our estimates of the number of patients who suffer from the diseases we are targeting and the number of patients that may enroll in our clinical trials; the ability to commercialize our product candidates, if approved; our ability and the potential to successfully manufacture and supply our product candidates for clinical trials and for commercial use, if approved; our ability to accurately forecast future financial results and timelines; our anticipated use of our existing resources, our estimates regarding expenses, future revenue, capital requirements and needs for additional financing and our ability to obtain additional capital; the sufficiency of our existing cash and investments to fund our future operating expenses and capital expenditure requirements; the potential diminishing need for therapeutics to address COVID-19; our ability to attract and retain qualified personnel; the implementation of our business model and strategic plans; the scope of our intellectual property protections we are able to establish and maintain; our ability to contract with third-party suppliers and manufacturers and their ability to perform adequately; developments relating to our competitors and our industry, including competing product candidates and therapies; general economic and market conditions; and other risks described in our public filings with the Securities and Exchange Commission, including our most recent Quarterly Report on Form 10-Q filed on November 3, 2022. New risk factors emerge from time to time, and it is not possible for our management to predict all risk factors, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in, or implied by, any forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Additionally, statements that “we believe” and similar statements reflect our management’s beliefs and opinions on the relevant subject. These statements are based on information available to us as of the date hereof, and although we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted a thorough inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain, and readers are cautioned not to unduly rely upon these statements. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason. This presentation includes information on drug candidates that are under clinical investigation, and which have not yet been approved for marketing by the U.S. Food and Drug Administration. The drug candidates are currently limited by federal law to investigational use, and no representation is made as to their safety or effectiveness for the purposes for which they are being investigated. Igm biosciences® 2

Global leaders in engineering and manufacturing IgM antibodies IgM antibodies have structural attributes that provide advantages relative to traditional IgG antibodies in multiple applications Agonist antibodies T cell engaging antibodies Targeted cytokine delivery We plan to extend our global leadership position in IgM antibodies in: Clinical development Manufacturing Research Intellectual property Cash and investments of $427.2 million (unaudited) as of December 31, 2022 Global leaders in the development of IgM antibodies Autoimmunity and Inflammation Oncology Infectious Diseases IGM Biosciences Overview Igm biosciences® 3

Additional binding sites lead to: Superior total binding power (avidity) Increased cross-linking of receptors for greater agonism IgM antibodies have unique structural attributes compared to IgG antibodies IgG IgM 2 BINDING SITES 10 BINDING SITES J-Chain allows for novel bi-specifics IgM core can enable complement engagement or inactivation Igm biosciences® 4

Strategic priorities for 2023 Accelerate development of our agonist Death Receptor 5 IgM antibody (IGM-8444) Develop our IgM T cell engager antibodies in autoimmune disease Extend our clinical pipeline Continue to develop our IgM antibody platform Igm biosciences® 5

Strong DR5 activation requires multi-receptor agonism DR5 is highly expressed across many different tumor types In vitro apoptosis comparing IgG and IgM DR5 antibodies using the same binding domain IgG IgM Ligand Apoptosis Cell Death Weak Apoptosis Cell Death Strong Apoptosis Cell Death [BAR_CHART]Igm biosciences® 6

IGM-8444 (5 mg/kg Q2D x 7) Irinotecan (100 mg/kg QW x 3) IGM-8444: activity seen in combination with irinotecan (in vivo) One of the active agents in FOLFIRI chemotherapy treatment [BAR_CHART] Igm biosciences® 7
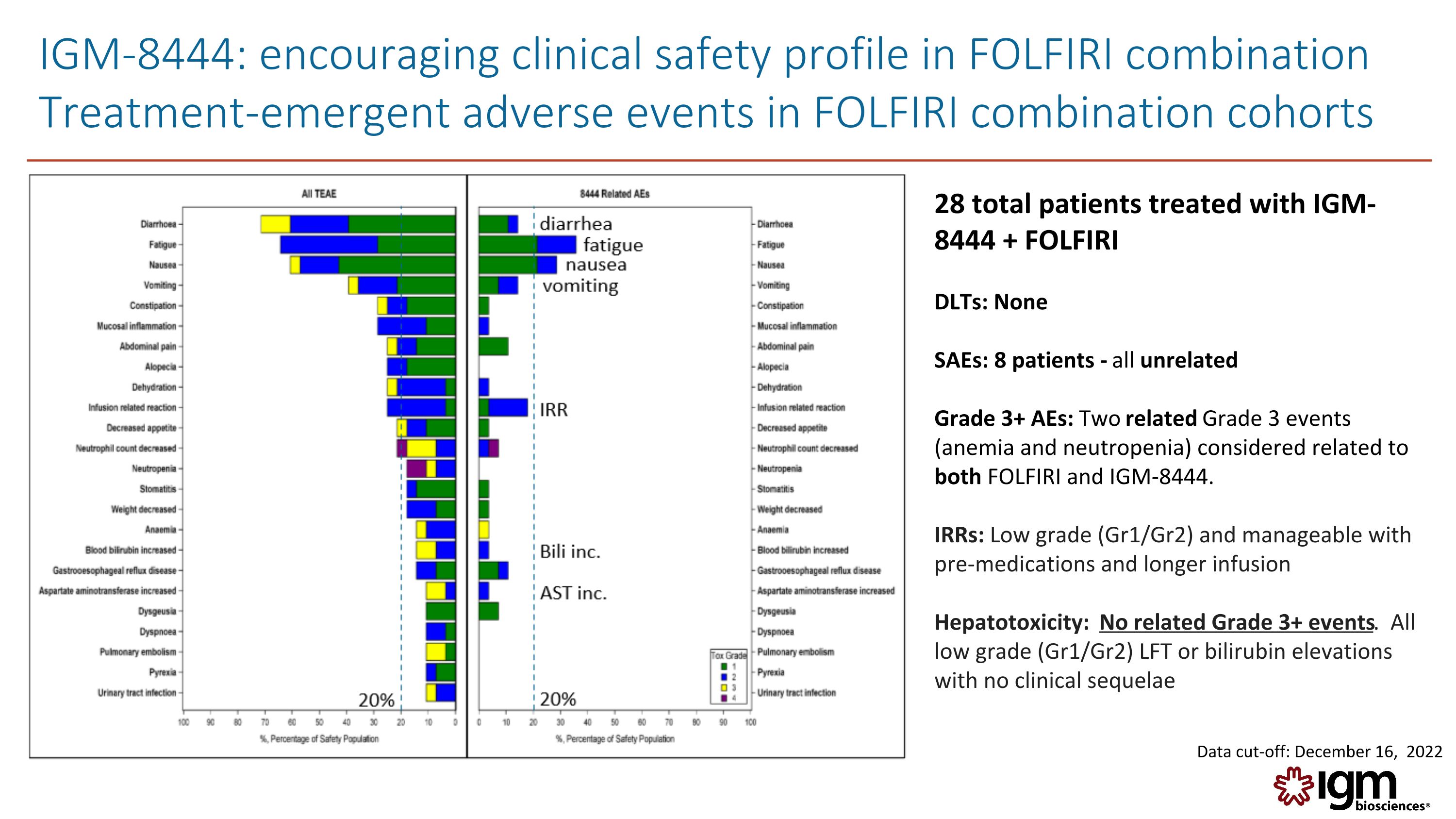
IGM-8444: encouraging clinical safety profile in FOLFIRI combination Treatment-emergent adverse events in FOLFIRI combination cohorts fatigue IRR vomiting nausea 20% diarrhea 20% AST inc. Bili inc. 28 total patients treated with IGM-8444 + FOLFIRI DLTs: None SAEs: 8 patients - all unrelated Grade 3+ AEs: Two related Grade 3 events (anemia and neutropenia) considered related to both FOLFIRI and IGM-8444. IRRs: Low grade (Gr1/Gr2) and manageable with pre-medications and longer infusion Hepatotoxicity: No related Grade 3+ events. All low grade (Gr1/Gr2) LFT or bilirubin elevations with no clinical sequelae Data cut-off: December 16, 2022 [BAR_CHART] Igm biosciences®

IGM-8444: Encouraging efficacy profile in colorectal cancer (CRC) combination. Strong rationale for randomized CRC study with FOLFIRI 9 All CRC patients treated in second through fourth FOLFIRI combo cohorts who are time eligible for second scan by data cut-off Best percentage change in tumor size PR (4L) (4L) (3L) (3L) (4L) (3L) (2L) (3L) (2L) (2L) (3L) (3L) (1L) PR PR Sx PR 1 mg/kg 3 mg/kg 10 mg/kg DOSE Prior FOLFIRI treatment All prior FOLFIRI patients showing tumor shrinkage progressed or had stable disease as best response to prior FOLFIRI treatment 3L+ Median PFS: 5.5 months (9 patients) Longest 3L+ progression free survival 12+ months 3L CRC SOC: PFS ~2 months, OS ~7 months 13 patients (9 at 3 mg/kg) Data cut-off: December 31, 2022 SOC-standard of care; PFS-progression-free survival; OS-overall survival; Sx – Surgery; PR – Partial Response [BAR_CHART] Igm biosciences® 9

Selected patient profiles: substantial benefit observed in patient's refractory to prior FOLFOX/FOLFIRI treatment Data cut-off: December 31, 2022 *Response Confirmed 69, mCRC, MSS, KRASmt, BRAFwt Prior FOLFIRI progression Prior Treatments Time on Tx Outcome Xeloda 5m FOLFIRI + bev 5m SD PD XELOX 2m SD PD IGM-8444 + FOLFIRI 12m+ PR (-36%)* 47, mCRCMSI-low, BRAFwt, KRASmt (G12D) Prior FOLFIRI progression Prior Treatments Time on Tx Outcome FOLFOX6 Xeloda/XRT 2 + 1m FOLFIRI + bev 5m NED PD FOLFIRI + bev 3m SD PD Clinical Trial 2m PD IGM-8444 + FOLFIRI 8.3 PR (-85%)* 52, mCRC, MSI-low, KRASwt, BRAFwt Prior Treatments Time on Tx Outcome FOLFOX 6m NED PD IGM-8444 + FOLFIRI 5m (-28%) Treatment qualified patient for curative surgery 63, mCRC, NRASmt, KRASwt, BRAFwt, Prior FOLFIRI experience Prior Treatments Time on Tx Outcome FOLFOX6 6m SD PD FOLFIRI + bev 2m SD SD IGM-8444 + FOLFIRI 5.8m PR (-48%)* *Response Confirmed *Response Confirmed bev-bevacizumab; m-mths; SD-stable disease; PD-progressive disease; NED-no evidence of disease Igm biosciences® 10
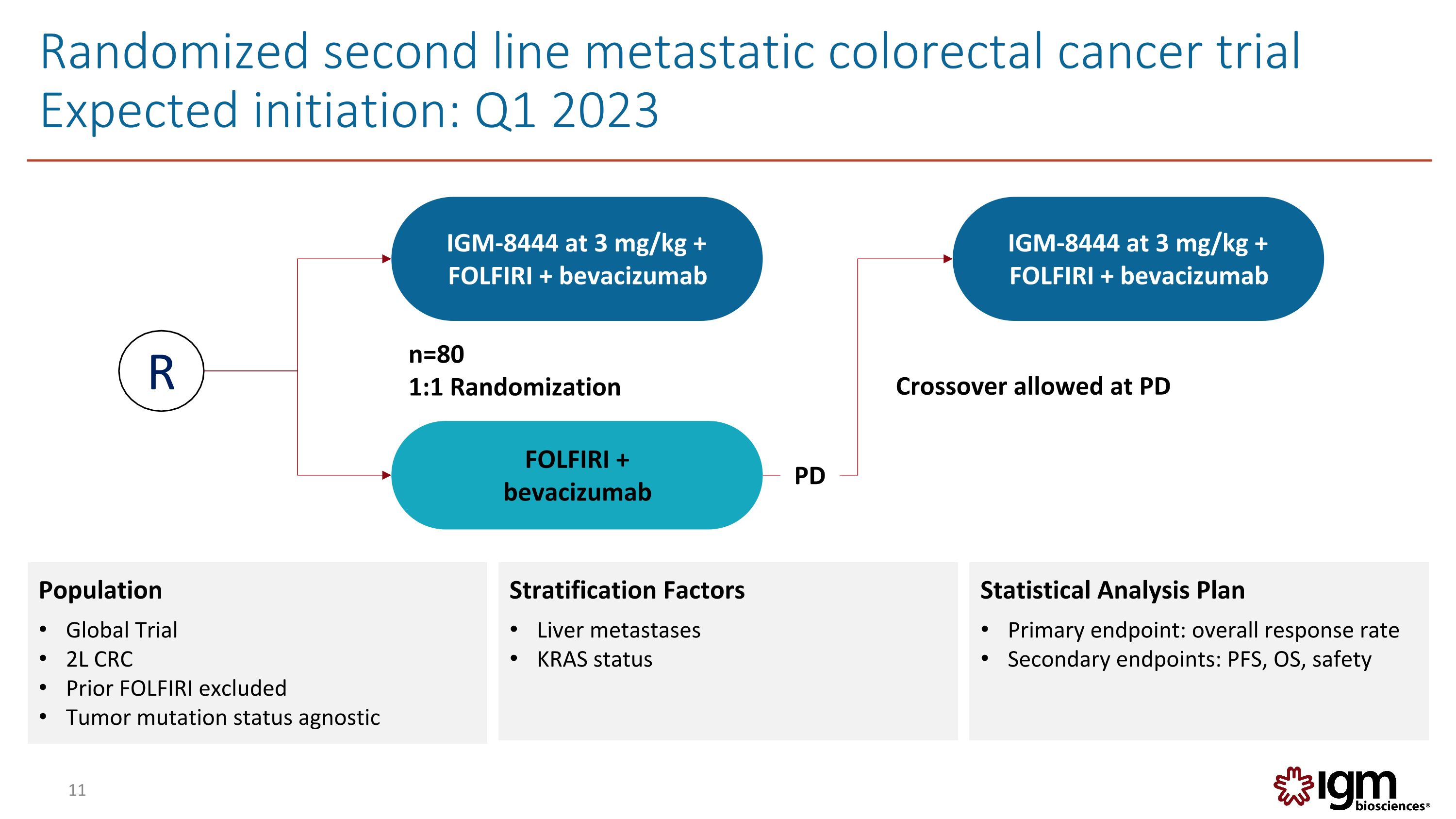
Randomized second line metastatic colorectal cancer trial Expected initiation: Q1 2023 11 FOLFIRI + bevacizumab n=80 1:1 Randomization IGM-8444 at 3 mg/kg + FOLFIRI + bevacizumab R Crossover allowed at PD IGM-8444 at 3 mg/kg + FOLFIRI + bevacizumab PD Population Global Trial 2L CRC Prior FOLFIRI excluded Tumor mutation status agnostic Stratification Factors Liver metastases KRAS status Statistical Analysis Plan Primary endpoint: overall response rate Secondary endpoints: PFS, OS, safety Igm biosciences® 11

Initial focus of IGM-8444 development: improving FOLFIRI + bevacizumab, the most common regimen for 2L colorectal cancer 1L 2L 3L+ Current Standard of Care FOLFOX (or other 5-FU chemo combos) + targeted agents FOLFIRI (or other 5-FU chemo combos) ± targeted agents Various Current Standard of Care Bevacizumab EGFR mAb (RASwt only) Pembrolizumab (MSI-H only) Bevacizumab EGFR mAb (RASwt only) Regorafenib Trifluridine/tipiracil Other Chemotherapy Efficacy Benchmark ORR: ~55% mPFS: ~10+m mOS: 24+ mos ORR: 5-20% mPFS: ~ 6 m mOS: ~ 12 m ORR: 1-2% mPFS: ~ 2 m mOS: ~ 6 m US Patient Incidence* ~50,000/year ~30,000/year ~20,000/year Limited effectiveness of later line therapies: low ORR and survival durations * Source: GlobalData accessed October 2022 Igm biosciences® 12

Synergistic activity seen in combination with other apoptotic pathway drugs: venetoclax and birinapant (in vivo) Triple Negative Breast Cancer Model (MDA-MB-231) Source: Sinclair, A. "Multimeric IgM antibodies targeting DR5 are potent and rapid inducers of tumor cell apoptosis In Vitro and In Vivo" PEGS Europe, November 2021. Data on file. DR5: extrinsic apoptotic pathway Birinapant: intrinsic apoptotic pathway DR5: extrinsic apoptotic pathway Venetoclax: intrinsic apoptotic pathway [BAR_CHART] Igm biosciences® 13

Additional IGM-8444 ongoing combination studies Data cut-off: December 16, 2022 Additional Combination Cohorts IGM-8444 + Birinapant IGM-8444 + Venetoclax Planned Activities 4th dose cohort currently being tested No DLTs observed Solid tumors, all comers 1st cohort open in SLL/CLL 1st cohort open in AML (venetoclax plus azacytidine) Igm biosciences® 14

Strategic priorities for 2023 Accelerate development of our agonist Death Receptor 5 IgM antibody (IGM-8444) Develop our IgM T cell engager antibodies in autoimmune disease Extend our clinical pipeline Continue to develop our IgM antibody platform Igm biosciences® 15

Using T cells to treat autoimmune disease via deep B cell depletion Recent proof of concept data showed extensive B cell depletion using an anti-CD19 CAR-T can treat autoimmune patients and potentially reset their immune systems 5 patients with treatment refractory systemic lupus erythematosus (SLE) treated with anti-CD19 CAR-T Resulted in durable, drug-free remission and shift to more naïve immunophenotype Depletion of pathogenic immune cells deep within the tissues appears to be critical to this result Mackensen et al., Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus, Nature Medicine, October 2022. [BAR_CHART] Igm biosciences® 16

T cell engagement with imvotamab can enable deep B cell depletion Imvotamab non-human primate cross reactive surrogate achieves >85% reduction of CD20+ B cells in spleen, mesenteric lymph nodes and bone marrow Deep depletion of CD20+ cells within tissue (in vivo) More effective depletion of low CD20 expressing cells than rituximab or IgG CD20 x CD3 (in vitro) Antibody (pM) Imvotamab IgG CD20 x CD3 Rituximab Deep B cell depletion in tissues and depletion of low CD20 B cells [BAR_CHART] Igm biosciences® 17

Opportunity for IgM T cell engager antibodies in autoimmune disease CD20 targeted antibodies are used with varying degrees of success in a broad range of autoimmune diseases, including systemic lupus erythematosus (SLE), rheumatoid arthritis and multiple sclerosis Monospecific anti-CD20 antibodies deplete circulating B cells, but are less effective in depleting immune cells in tissues where NK cells and complement are less available Reservoirs of pathogenic auto-antibody producing cells can persist and drive further disease Imvotamab has shown an advantageous safety profile among CD20 x CD3 T cell engagers We plan to file INDs in 2023 for imvotamab in multiple autoimmune diseases First IND is planned to be in SLE IGM-2644 (CD38 x CD3) may also provide an alternative or additional approach to immune cell depletion Igm biosciences® 18

Strategic priorities for 2023 Accelerate development of our agonist Death Receptor 5 IgM antibody (IGM-8444) Develop our IgM T cell engager antibodies in autoimmune disease Extend our clinical pipeline Continue to develop our IgM antibody platform Igm biosciences® 19

Imvotamab oncology clinical development Arm A: 15/50/100 mg titration dose Arm B: 15/50/100/300mg titration dose Monotherapy dose finding studies Potential combination studies * Combination will start with a full dose of loncastuximab with 100 mg of imvotamab R imvotamab + loncastuximab R/R NHL* Dose selection: 100mg vs. 300mg DLBCL and Follicular Lymphoma Up to 30 patients per arm, per disease Dose finding in DLBCL expected Q2 2023 Imvotamab + loncastuximab study expected to begin in Q1 2023 Additional combination studies under consideration imvotamab + tafasitamab + lenalidomide Imvotamab + immunomodulatory drugs Igm biosciences® 20

Imvotamab plus loncastuximab: increased potency (in vivo) Two tumor targets, two mechanisms of action: reduced chance for escape % Tumor growth inhibition Efficacy model is Raji s.c. with human PBMC engraftment 5 mg/kg imvotamab q2dx12 i.v. starting on day 5 0.1 mg/kg loncastuximab is a single i.p. dose on day 5 (prior to imvotamab) [BAR_CHART] Igm biosciences® 21

IGM-7354: Targeted IL-15 delivery via PD-L1 expressing cells NK or CD8 T cell expansion IGM - 7354 IGM-7354 presents IL-15 to CD8 and NK cells Targeted delivery may increase efficacy and reduce toxicity First patient dosing expected: Q1, 2023 Tumor and/or Antigen Presenting Cell IL-15/IL-15R x anti-PDL1 IgM Anti-PD-L1 binder IL-15R/IL-15 Igm biosciences® 22

Immune stimulation with IGM-7354 Adapted from Giffon et al., AACR 2022 1 mg/kg Vehicle 3 mg/kg 10 mg/kg 1 mg/kg Vehicle 3 mg/kg 10 mg/kg (in vivo) (in vivo) [BAR_CHART] Igm biosciences® 23

Expansion of T Cell engager platform beyond imvotamab IGM-2644 CD38 x CD3 Multiple Myeloma Autoimmunity Multiple Myeloma Phase 1 study initiation expected Q1 2023 Indications Timing IGM-2537 CD123 x CD3 AML MDS ALL AML Phase 1 study initiation expected H2 2023 Igm biosciences® 24

IGM-2644 (CD38 x CD3) shows robust activity in pre-clinical models Differentiated potency against low CD38 expression cells (in vitro) CD38 expression level [BAR_CHART] Igm biosciences® 25

IGM-2644: reduced cytokine release relative to bispecific IgG (in vitro) Bispecific IgG IGM-2644 IGM-2644 IGM-2644 IGM-2644 Bispecific IgG Bispecific IgG Bispecific IgG [BAR_CHART] Igm biosciences® 26

IGM-2537 (CD123 x CD3): Robust activity in acute myeloid leukemia model (in vivo) MV4-11 AML Xenograft Model [BAR_CHART] Igm biosciences® 27

IGM-2537: Reduced cytokine release relative to bispecific IgG (in vitro) Bispecific IgG Bispecific IgG MV4-11: IL-2 release MV4-11: IL-4 release MV4-11: IFNγ release Reduced cytokine release compared to bispecific IgG [BAR_CHART] Igm biosciences® 28

Strategic priorities for 2023 Accelerate development of our agonist Death Receptor 5 IgM antibody (IGM-8444) Develop our IgM T cell engager antibodies in autoimmune disease Extend our clinical pipeline Continue to develop our IgM antibody platform Igm biosciences® 29

Next Generation: Co-stimulatory IgM T cell engagers Two activation signals within single immune synapse Enhanced T cell activation CD28 or 41BBL co-stimulation provides increased T cell activation and proliferation for sustained anti-tumor response IgM platform allows dual-engagement while maintaining full avidity advantage for tumor targeting Potential for responses in “cold” solid tumor setting with low T cell counts Signal 1: CD3 Signal 2: CD28 or 4-1BBL Igm biosciences® 30

T cell co-stimulation can create significantly enhanced T cell activation (in vitro) Signal 1+2 Signal 1 Signal 2 CD28 co-stimulation with CD3 (T-cell activation) 4-1BBL co-stimulation with CD3 (T-cell Activation)* *T cell activation uses a 4-1BB expressing reporter cell activation assay [BAR_CHART] Igm biosciences® 31

Novel therapeutic approaches to address infectious diseases IgMs evolutionarily designed as “the first line of humoral defense” Opportunities to maximize therapeutic potential Differentiated applications in infectious diseases “Virus trapping” antivirals Aerosolized/inhaled therapeutics Affinity engineering to address specific targets J-chain facilitates mucosal transport to sites of initial infection High avidity can address viral variation First antibody defense upon pathogen infection Avidity provides broad protection against pathogens Stronger complement fixation than IgG antibodies Igm biosciences® 32

Viral trapping strategy: IgM structure/avidity enables virus neutralization by “trapping” the virus using its cellular receptor IgM core Molecular construction ACE2 receptor (cellular receptor for SARS viruses) ACE2-decamer ACE2-decamer is ~100x more potent than bivalent ACE2-IgG-Fc in neutralizing Omicron and other variants (in vitro) Valency of IgM enables potent virus neutralization Antibody Conc (ng/ml) ACE2-decamer ACE2-IgG-Fc [BAR_CHART] Igm biosciences® 33

IgM virus trapping is a novel antiviral strategy designed to be resilient against viral evolution Antiviral potency of ACE2-decamer designed to be retained despite viral mutations SARS-family viruses may not be able to change dependence on their sole receptor (ACE2) for infection Designed to improve potency as virus evolves to increase ACE2 receptor binding affinity SARS-CoV-1 (Urbani strain) neutralized with similarly high potency ACE2-decamer is designed to neutralize all viruses that rely solely on the ACE2 receptor for entry More Potent Consistently high potency across SARS-COV2 variants *Sotrovimab initially approved under EUA in 2020 but removed due to reduced potency against variants IC50 (ng/mL) in pseudovirus assays 2020 2021 2022 ACE2-decamer Sotrovimab (S309) [BAR_CHART] Igm biosciences®

Sanofi/IGM multi-target collaboration agreement $150M upfront payment received Equity investment in April 2022 follow-on offering Potentially $6B+ in preclinical, clinical, regulatory and commercial milestone payments Sanofi responsible for worldwide commercialization Financial Terms Global research collaboration to leverage proprietary IgM antibody technology platform to create, develop and commercialize agonists against three oncology targets and three autoimmunity and inflammation targets Igm biosciences® 35

Multiple catalysts anticipated over next eighteen months Clinical Data and Pipeline Expansion Igm biosciences® 36 Clinical Data and Pipeline Expansion IGM-8444 (DR5) Initial randomized IGM-8444 + FOLFIRI + bevacizumab data in 2L colorectal cancer patients Imvotamab (CD20 x CD3) Initial clinical data in autoimmune patients Initial loncastuximab combination data IGM-2644 (CD38 x CD3) Initial multiple myeloma data Initiate clinical testing in autoimmune patients IGM-7354 (IL15 x PD-L1) Initial clinical data in solid tumor patients IGM-2537 (CD123 x CD3) Initiate clinical testing in AML patients

Global Leaders in IgM Antibodies Igm biosciences® REIMAGINING antibody medicines
